What is the endosure test?
The EndoSure Test is a non-invasive, diagnostic test for endometriosis. It is easy to perform and requires a reclining bed/chair and a quiet room. The test takes around 60 minutes after which time the result is available for the clinician to interpret.
- The test will detect endometriosis and adenomyosis.
- It will detect thoracic endometriosis.
- It will detect all stages of endometriosis.
- It will detect endometriosis not visible on transvaginal ultrasound and MRI.
- Removes the human judgement required in a diagnostic laparoscopy.
- It is very accurate, 95% +
- Measures the level of activity of endometriosis.
- Can be used as an initial test.
- Can be used for post-surgical and post-medical treatment monitoring.
- Allows for early diagnosis and selection of patients who will respond to medical therapy.
- Early treatment will suppress disease, prevent advancement and lead to lower surgery rates.
How is the endosure test performed?
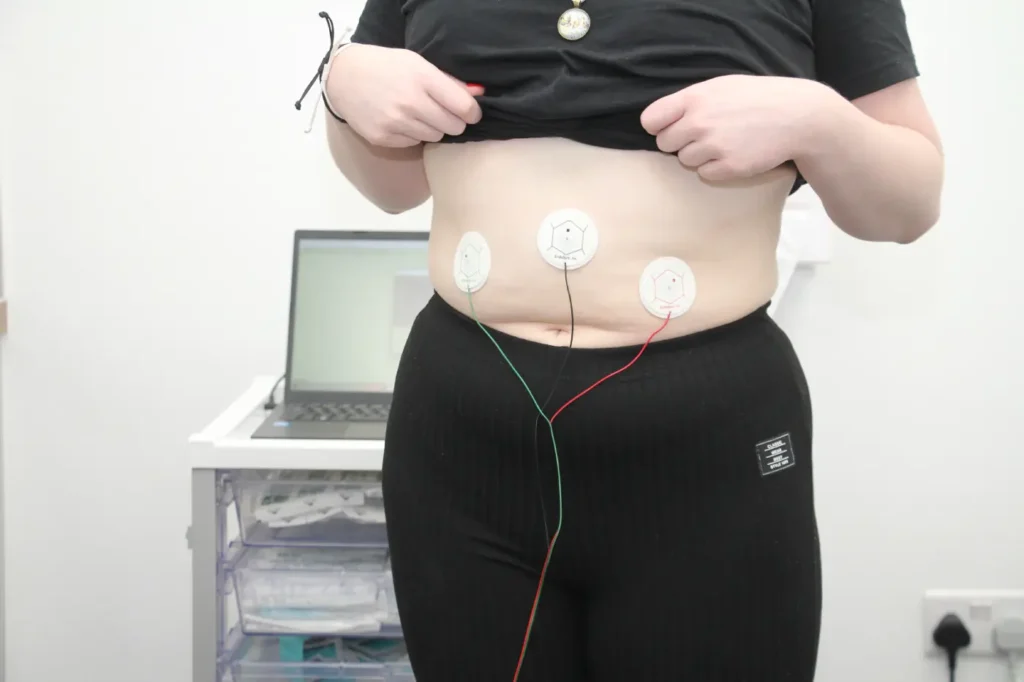
- The patient lies on a reclining bed or chair in a quiet room
- Three electrodes are placed on the abdomen
- A ten-minute baseline recording of the myoelectrical activity of the bowel is made.
- The patient drinks water until feeling completely full.
- A thirty-minute post stimulation recording is made.
- The results are available within ten minutes.
Activity and clinical feedback
We have performed several hundred tests in the U.K. so far. These have been in the following areas:
- As an initial test in patients presenting with abdominal pain.
- In post-treatment monitoring. Patients that have had a surgical clearance of endometriosis but who present with pain months/years after surgery.
- As a diagnostic aid in patients who have had a negative laparoscopy but when pain is still suspected to be endometriosis/ adenomyosis.
- Testing of patients with fertility issues.
Benefits of the endosure test
At the current time, patients typically wait 8 years, seeing 10-15 practitioners from the time of symptom presentation until a diagnosis of endometriosis is confirmed. During this time the condition will have worsened. For many, this results in a diagnostic laparoscopy followed by extensive and radical surgical excision. This is in addition to time lost in advancement of academic and professional pursuits with massive loss in productivity.
The EndoSure Test will permit early diagnosis and suppression treatment which will reduce the number of surgeries, fruitless visits to practitioners, and unsuccessful medical and radiological tests going forward. This will have an immediate and significant reduction in cost to both the NHS and private healthcare insurance groups.
The EndoSure Test answers perfectly the harsh criticisms of the recently published report of the Women and Equalities Select Committee Report published on 11 December 2024.
Historical Timeline
-
1998
The EndoSure Test was introduced in 2022 however; the scientific discovery was first reported back in 1998 by John Mathias. His paper entitled Relation of endometriosis and neuromuscular disease of the gastrointestinal tract: new insights, was published in Fertility and Sterility.
-
2004
Development of the Electroviscerogram (EVG) and confirmation of the device’s ability to detect the same GIMA biomarker noted in transoral studies of Mathias. Regulatory documentation created.
-
2007
Clinical deployment of the Electroviscerogram (EVG). It was developed to non-invasively detect the same high frequency small bowel pattern as the antral-duodenal motility in the 1998 paper. It uses gastrointestinal myoelectrical activity (GIMA)
-
2007 - 2015
Technology validation studies. Over 4500 patients were studied to validate the software and hardware. “Normal” GIMA signal patterns were established in females as well as males. A unique signal pattern for endometriosis was confirmed in real world experience. Documented that no other diseases had similar biomarker fingerprint to endometriosis and adenomyosis.
-
2015 - 2017
Formal studies were conducted. Confirms Normal v Endometriosis related GIMA and proves the concept in a pivotal, blinded, surgically verified 154 Women Trial. The study was approved by the Ethics Committee of Woman’s Hospital of Texas.
-
December 2022
Mark Noar presents to the American Association of Gynaecologic Laparoscopists. (AAGL) Ai-Derived Threshold Score of Intraabdominal Myoelectrical Activity Predicts Prescence and Stage of Endometriosis with 100% Accuracy-Electroviscerography (EVG) in Endometriosis. Mark D. Noar MD, MPH, John Mathias MD.
-
June 2023
UKAS and MHRA approval granted
-
May 2024
Study published in the Journal of Clinical Medicine. Gastrointestinal Myoelectrical Activity (GIMA) Biomarker for Non-invasive Diagnosis of Endometriosis. Mark Noar, John Mathias, Ajit Kolatkar.
-
November 2024
Publication in the Journal of Gynaecology and Obstetrics, Volume 14, Issue 5, No:626. Validation of New GIMA Biomarker Signature of Endometriosis-Interim Data: Research Article. Mark Noar, John Mathias, Ajit Kolatkar.
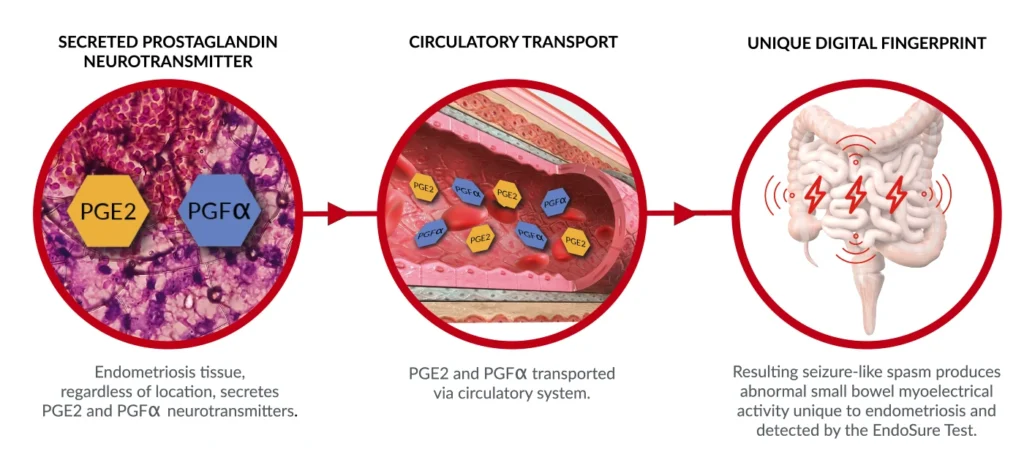
What Is The Background To The Endosure Test ?
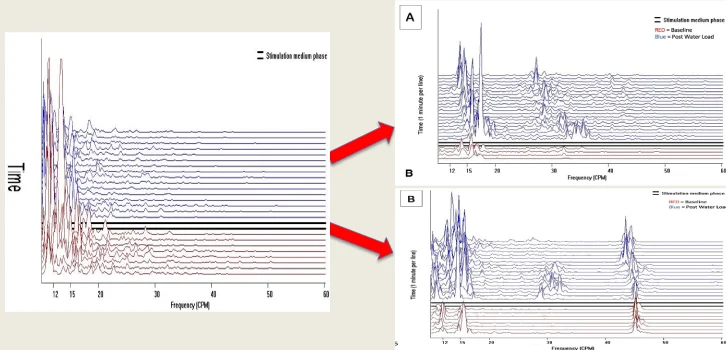
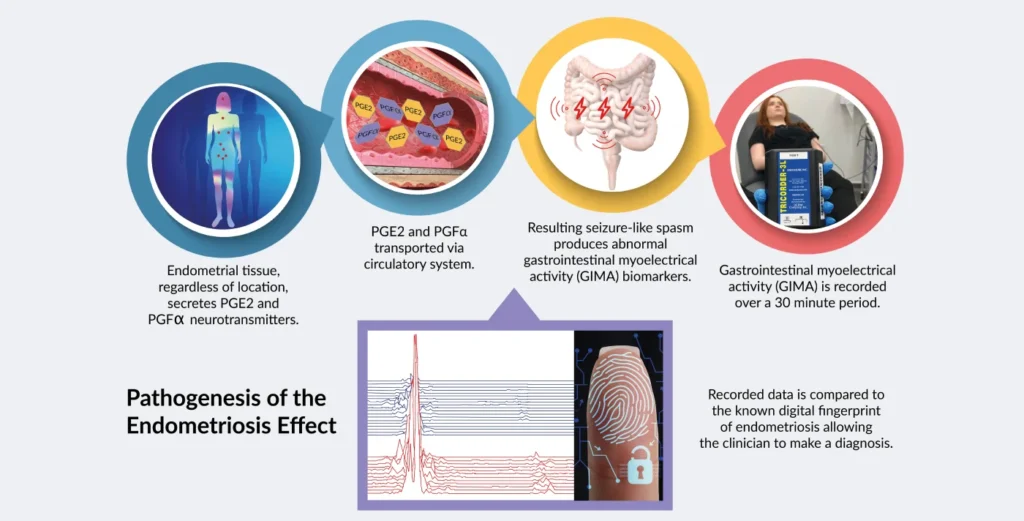
The science behind the test was initially published in the gynaecological literature in 1998. The discovery revealed that endometrial tissue releases large simultaneous amounts of prostaglandin PGE2 and PGFa, which have a seizure-like effect on the motility of the small bowel. This activity is known as gastrointestinal myoelectrical activity and is a newly
described biomarker called the GIMA Biomarker. The GIMA Biomarker is detected by the currently approved device known as the Electroviscerogram or EVG. The EVG collects, records, and interprets the
signal which is unique and present only in subjects with endometriosis or adenomyosis, and not found with any other diseases.